A simplified and automated ISO 13485 certification
Optimiso Suite is a precious help to ensure ISO 13485 certification and thus guarantee the quality of your medical devices. Information is centralized, updates automated and you control the costs associated with certification.
Both powerful and intuitive, the software combines the rigor of a quality system with user-friendly access to information.
- Automatic update and centralized information
- Risk management and monitoring of controls automated
- Compliance management and audits made easy

Automatic update and centralized information
With Optimiso Suite, quality documentation for medical devices is centralized on a single platform: processes, procedures, documents (medical device file, warning sheets, etc.), non-conformities, complaints, audits, risks, controls, responsibilities, skills, corrective and preventive actions, etc. The software automatically draws diagrams and flowcharts.
Equipped with a powerful system of links, it guarantees consistency and continuous updating of data. You modify an element and the update is automatically reflected on all the quality system. The software ensures the security and integrity of the recordings. All changes are tracked and the different versions logged. Data security is guaranteed and confidential medical information is protected.
In addition, Optimiso Suite makes it easy to manage QHSE – Quality, Hygiene, Security and Environment – systems.
- Automatic impact of any modification
- Information traceability
- Consistent and error-free QMS
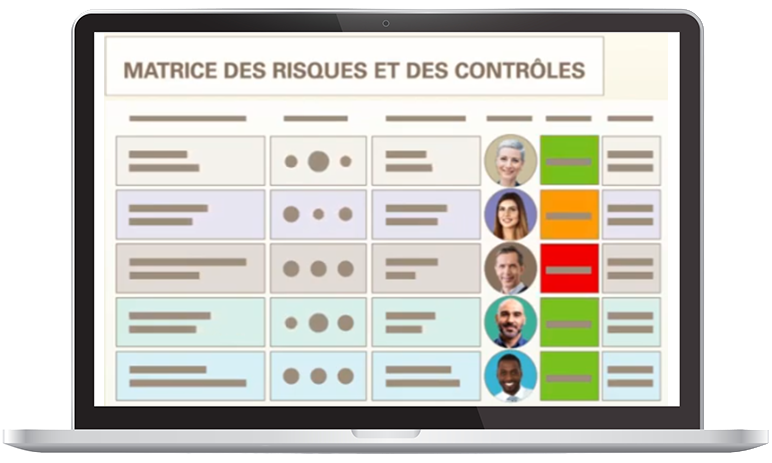
Risk management and monitoring of controls automated
Optimiso Suite makes it easy to analyze and assess risks related to the safety and performance of medical devices. Linked to all the elements of the quality system, the risks are easily readable in an always up-to-date matrix and mapping.
The monitoring of controls, surveillance and control of equipment are fully automated.
Optimiso Suite provides a real-time view of the status of controls. Employees are alerted to the controls to be carried out and management is informed of the results.
- Intuitive reading of risks and controls
- Monitoring the status of controls in real-time
- Traceability of controls

Compliance management and audits made easy
Optimiso Suite brings time-saving and serenity to the Quality Manager. Preparing for the audit is made easier because the auditor has access to all documentation on a single website, including old versions, modes of evidence and records.
Thanks to digital audits, findings, non-conformities, corrective and preventive actions are easier to manage.
In addition to the ISO 13485 standard, Optimiso Suite makes it possible to integrate all the regulatory requirements applicable in all countries where medical devices are marketed. The compliance report makes it easy to verify that these requirements are met.
- Automatic follow-up of recommendations and non-conformities
- Immediate access to all documentation, methods of proof and records
- Efficient support to meet regulatory and normative requirements
How can we help you?
Read informations and best practices
- QSE software in SaaS: 20 criteria for a comparison [Read more]
- Understand the difference between process and procedure [Read more]
Price for the ISO 13485 software
Some examples of prices for Optimiso Suite in Cloud mode
The fundamentals for getting started: describe and communicate the processes, procedures and other documents.
With the modules :
from
292 € excl. VAT per month
for 1 editor
and up to 10 readers
Ideal for further digitizing the QMS and managing incidents, improvements and risks.
With the modules of the Basic Pack and
◊ Incidents
◊ Improvements
◊ Risks
from
446 € excl. VAT per month
for 1 editor
and up to 10 readers
To go further and integrate mitigation measures, indicators, skills, missions, compliance with the standard, etc.
With the modules of the Standard Pack and
◊ Controls
◊ Indicators
◊ Skills
◊ Missions
◊ ToDo Manager
◊ Business Intelligence
◊ Standards & Laws
◊ Assets
from
1’080 € excl. VAT per month
for 1 editor
and up to 10 readers

A consulting service at your disposal
Benefit from consultants with extensive practical experience in ISO 13485 certification to support you in your project. Discover our consultancy services here.
Choose Optimiso Suite as you wish
In Cloud mode (SaaS) to free you from any technical constraints or in license mode (On-premise) to keep control of your software environment.

A quality support
Benefit from both technical and business experts who have implemented similar projects. In addition, you benefit every month from good practices in video or image as well as a support platform with numerous training courses and FAQs.
How can we help you?

- Automatic update and centralized information
- Risk management and monitoring of controls automated
- Compliance management and audits made easy
